FDA Emergency Use Authorized, NOW WITH POINT-OF-CARE – Assure COVID-19 IgG/IgM Rapid Test Device
Instruction for Use (Assure, Fastep, Ecotest)
Fact Sheet for Healthcare Providers (Assure, Fastep, Ecotest)
Fact Sheet for Healthcare Recipients (Assure, Fastep, Ecotest)
FDA Letter of Authorization (Non-POC)
Instruction for Use (Non-POC)
Fact Sheet for Healthcare Providers (Non-POC)
Fact Sheet for Healthcare Recipients (Non-POC)
Quick Link: Product Complaint Form
What is Covid-19:
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
Indication
The Assure COVID-19 IgG/IgM Rapid Test Device is a rapid lateral flow chromatographic immunoassay intended for the qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in human venous whole blood (sodium EDTA), serum, plasma (sodium EDTA) and fingerstick whole blood. The Assure COVID-19 IgG/IgM Rapid Test Device is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity. The Assure COVID-19 IgG/IgM Rapid Test Device should not be used to diagnose acute SARS-CoV-2 infection.
Use of this test with all authorized specimen types is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. 263a, that meet requirements to perform moderate or high complexity tests.
This test is also authorized for use with fingerstick whole blood specimens only at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
Warning
▪ This test has not been FDA cleared or approved;
▪ This test has been authorized by FDA under an EUA for use by authorized laboratories;
▪ This test has been authorized only for the presence of IgM and IgG antibodies against SARS-CoV-2, not for any other viruses or pathogens.
▪ This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
▪ Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
▪ This product is intended for professional use and not for home use.
▪ Not for the screening of donated blood.
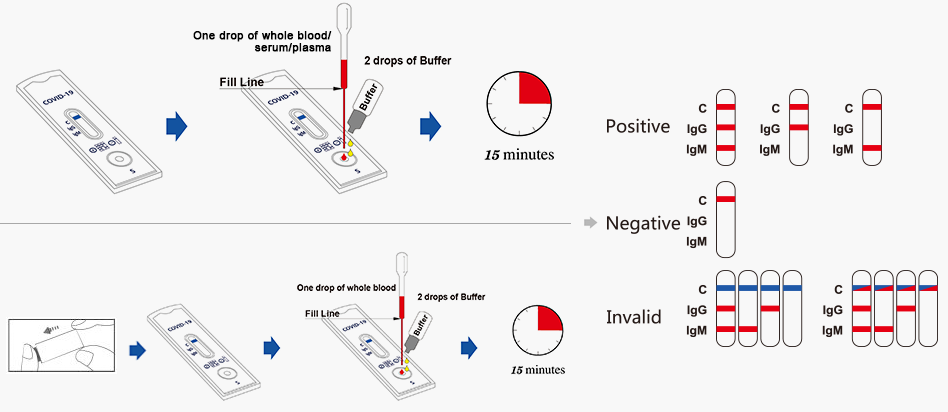
Test Procedure:
1. Bring the pouch to room temperature before opening. Remove the test device from the sealed pouch and use it as soon as possible.
2. Place the test device on a clean and level surface. Note: There should be a blue line in the control region (next to “C”), discard the device if there is no blue line.
3. Label the test with patient or control identification.
4. Add the specimens.
For Venous Whole Blood Specimens, Serum or Plasma Specimens
a) Using the provided disposable pipette, draw the specimen above the fill line (avoid the specimen entering the bubble of disposable pipette)
b) Transfer one drop of the specimen into the specimen well of the test device, then add 2 drops of buffer and start the timer. Adding more or less drops of specimen may lead to incorrect results. Adding 1 drop of buffer or more than 4 drops of buffer may lead to incorrect results.
For Fingerstick Whole Blood
a) Clean the puncture site with the alcohol prep pad provided.
b) carefully remove the cap from the safety lancet. Push the safety lancet firmly against the puncture site until it pricks the finger.
c) Using the provided disposable pipette, draw the specimen above the fill line(avoid the specimen entering the bubble of disposable pipette)
d) Transfer one drop (equivalent to 10L) of the specimen into the specimen well of the test device, then add 2 drops of buffer and start the timer. Adding more or less drops of specimen may lead to incorrect results. Adding 1 drop of buffer or more than 4 drops of buffer may lead to incorrect results.
Wait for the blue line change to red line, read results at 15 minutes, Do not read results earlier than 15 minutes or after 30 minutes.
Azure Biotech Inc. is a high-tech biotechnology company that specializes in research & development, production and sales of diagnostic reagents, POCT analyzer and biological materials… READ MORE